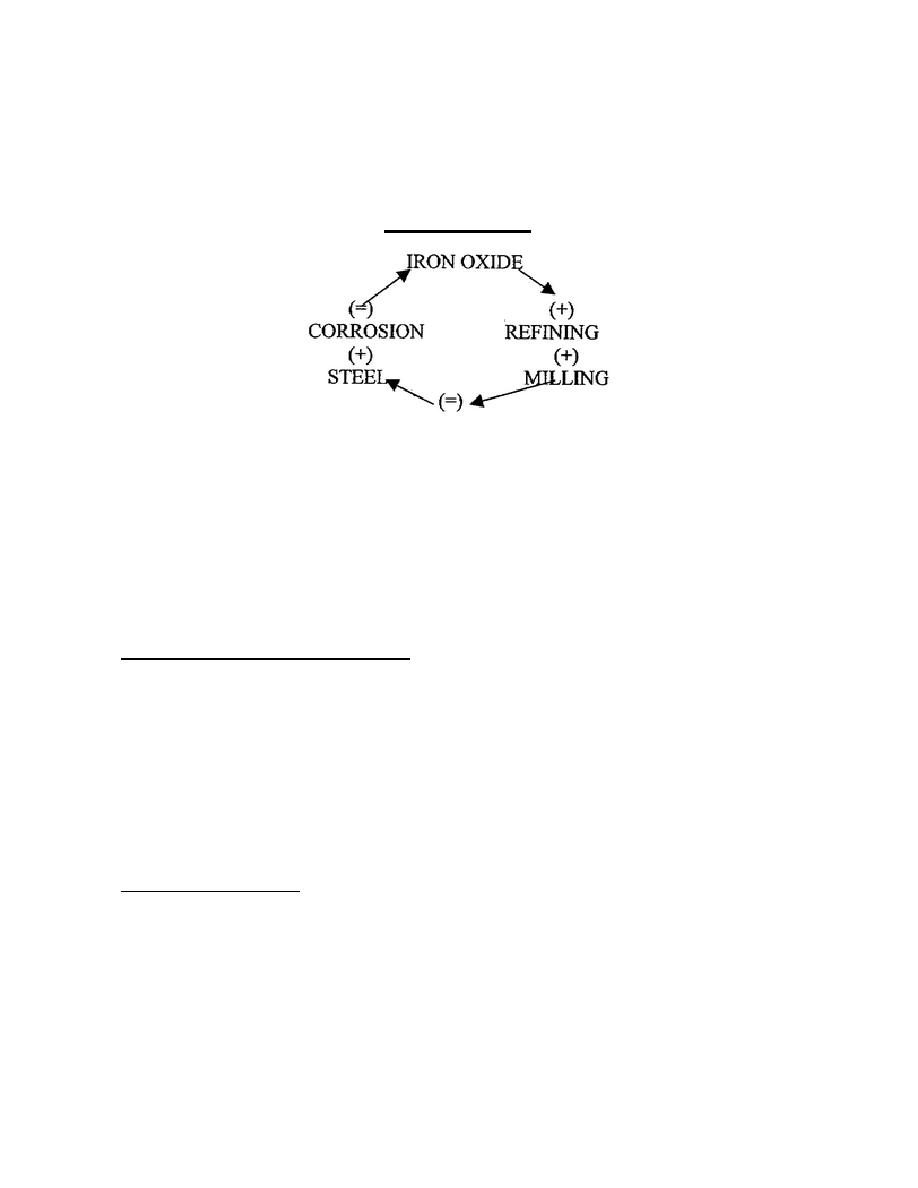
(2) To obtain metals from ores, the compounds are subjected to extreme heat to break the chemical
attractions between the elements.
(3) Energy is added through each step in the chain to shape the end product.
NOTE: The Corrosion Cycle; the material is constantly trying to return to its natural state.
The Corrosion Cycle
Figure 16-1.
b. Steel corrodes in either soil or water; electrical energy is released as this accrues. Corrosion is a
process that has two primary aspects.
(1) A physical change in the metal occurs.
(2) Direct electrical current (D-C) is generated.
(3) This process is referred to as an "ELECTRO-CHEMICAL" reaction.
2.
Conditions Necessary For A Corrosion Cell.
Electrolyte -- soil and/or water.
Anode -- deteriorates (corrodes).
Cathode -- protected by the anode.
External Connection -- Between the anode and cathode (pipe).
NOTE: All four of the above must be present for corrosion to proceed in a corrosion cell.
3.
Sources of Corrosion Cells.
Dissimilar Metals:
a. If any two materials shown are placed in a conducting environment, there will be a difference
between them.
QM5200
16-2



 Previous Page
Previous Page
